Click. Screen. Degrade.

University Frankfurt, University of Kiel, Ludwig-Maximilians-Universität
https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c02543
Direct-to-Biology (D2B) is a screening technique which combines plate-based synthetic chemistry with high-throughput screening using unpurified molecules. This allows for rapid synthesis and screening to identify new hits for a target or to expand SAR around existing molecules.
In this work, it was applied to PROTAC synthesis and screening, using a copper-catalysed azide–alkyne cycloaddition (CuAAC) to combine the POI ligands and E3 ligase warheads. The authors chose four proteins, which are well-established model systems for PROTAC development: Bromodomain-containing protein 4, soluble epoxide hydrolase, WD repeat-containing protein 5 and Aurora kinase A. This approach allowed hundreds of PROTACs to be synthesised and screened within a few days, with new hits identified for each target.
Unhooking the Hook: Optimization of the Aurora A Targeting PROTAC JB170 to CCT400028, an In Vitro Degrader Chemical Probe

The Institute of Cancer Research, University of Kiel https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c03024
The hook effect is a phenomenon in which increasing PROTAC concentration leads to decreased degradation, due to binary complex formation of POI-PROTAC or PROTAC-E3 Ligase. PROTACs which target Aurora A kinase, a protein that is overexpressed in many tumour types, suffer from a severe hook effect, as well as low selectivity and poor stability. Through modifications to the linker and rational optimisation of the CRBN warhead to decrease binary interactions with CRBN, the hydrolytic stability was increased while the hook effect was removed. This led to CCT400028, which also showed a superior degradation activity in all assays used, compared to the parent compound JB170. The researchers recommend CCT400028 for use as an in vitro tool compound to study Aurora A degradation, with work ongoing to develop compounds suitable for in vivo studies.
Identification of an allosteric site on the E3 ligase adapter cereblon

Harvard University, Scripps Research, GSK
https://www.nature.com/articles/s41586-025-09994-w
Cereblon (CRBN) is an E3 ubiquitin ligase substrate adapter protein. It is the primary target of thalidomide and related analogues, which bind at the orthosteric thalidomide-binding domain (TBD). Although compounds which bind to this domain have been thoroughly investigated, little is known about alternative binding sites on CRBN. In this work, SB-405483 was identified from a high-throughput screen using a time-resolved fluorescence energy transfer (TR-FRET) displacement assay. SB-405483 binds to an allosteric site and cooperatively enhances the binding of orthosteric ligands, altering their neosubstrate degradation profiles. The discovery of this compound and binding site opens up new opportunities for improving the selectivity and efficacy of CRBN therapeutics.
Parallels between the chloro and methoxy groups for potency optimization

Takeda Pharmaceuticals, Genesis Molecular AI https://pubs.rsc.org/en/content/articlelanding/2026/md/d5md00848d
The chlorine and methoxy substituents are two of the most common groups used in medicinal chemistry to decorate aromatic rings. Despite their opposing effects on the electron density of the aromatic rings they are bonded to, both groups are capable of dual electrostatic behaviour. This article discusses the main types of intermolecular interactions these groups participate in through a comprehensive search of the PDB and analysis of X-ray co-crystal structures.
Discovery and Characterization of Zelnecirnon (RPT193), a Potent and Selective CCR4 Antagonist for Allergic Disorders

RAPT Therapeutics
https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c01678
Zelnecirnon (RPT193) is a potent and selective CCR4 Antagonist for Allergic Disorders. The compound was developed by RAPT Therapeutics, who were acquired this month by GSK. The compound entered Phase I and II clinical trials, which were stopped due to unexpected liver toxicity in one patient, although the Phase I efficacy trials showed promise for this mechanism in treating atopic dermatitis. This drug annotation describes the development that led to Zelnecirnon and its subsequent characterisation.
Advances in BRET probes for intracellular target engagement studies

Promega, University of North Carolina at Chapel Hill, Johann Wolfgang Goethe University, Sloan Kettering Institute, University of Oxford, University of Alabama at Birmingham, University of North Carolina at Chapel Hill https://www.nature.com/articles/s41589-025-02103-y
Bioluminescence resonance energy transfer (BRET) assays are a powerful technique to measure target engagement in live cells. This perspective provides an update on the application of BRET technology for use in early-stage drug discovery and chemical biology, which goes beyond verification of intracellular target engagement
Metabolic Stability of Fluorinated Small Molecules: A Physical Organic Chemistry Perspective
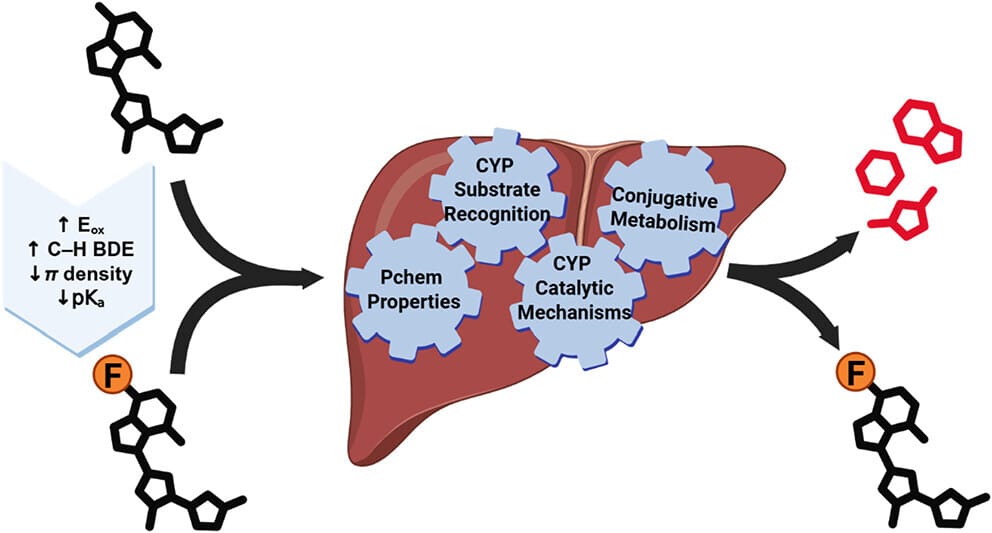
Purdue University
https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c02359
Fluorine atoms are one of the most used groups by medicinal chemists to improve a compound's metabolic stability, particularly to oxidative enzymes. The increase in metabolic stability is often attributed to the increased C-F bond strength relative to C-H bonds. In this perspective, the authors argue that this ignores the accepted mechanisms of drug metabolism. Instead, they explain the mechanisms by which oxidative metabolism occurs and the physical organic chemistry phenomena that enable fluorinated analogues to avoid those processes. They then apply these principles to matched molecular pairs of fluorinated and nonfluorinated drug candidates to assess their metabolic profiles.
Idler Compounds: A Simple Protocol for Openly Sharing Fridge Contents for Cross-Screening

University College London, UK Health Security Agency, University of Santiago de Compostela, Universitat de Barcelona https://pubs.acs.org/doi/10.1021/acs.jmedchem.5c02354
Any medicinal chemist knows how many compounds with great properties have been left to ‘gather ice in the freezer’ for one reason or another. In industrial settings, these compounds and their data cannot be shared due to ownership of intellectual property, while in academia, this is often less of a concern. This paper describes a simple method for sharing these forgotten compounds (termed “idlers”) with the aim of accelerating hit identification through serendipitous discovery.